Which of the Following Pairs of Compounds Are Isomers
Isomers have the same formula but different structures. Compounds in the second pair have different formulas.
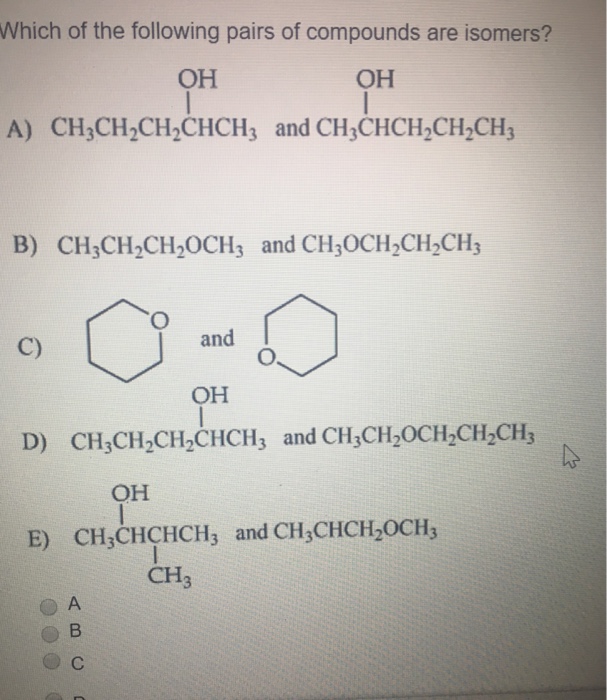
Solved Which Of The Following Pairs Of Compounds Are Chegg Com
Indicate whether the pair of structures shown represent stereoisomers constitutional isomers different conformations of the same compound or the same conformation of a compound viewed from a different perspective.
. In spite of having a similar subatomic equation the actual properties of every particle may vary especially if the. A cis-13-Dibromocyclohexane and trans-14-dibromocyclohexane b 23 -Dimethylhexane and 233 -trimethylpentane c FIGURE CANT COPY. Which of the following pairs of compounds are cis-trans isomers.
22 Which of the following pairs of compounds are cis-trans isomers. Correct option is B In FIgure I Fluorine and Iodine are cis to each other and in Figure II they are trans to each other hence they are pair of Geometrical isomers. I Ethanol and 2 propanol.
Interactive 3D display mode Interactive 3D display mode H₂C- CH HC-C1 H₂C- -c-a Interactive 3D display mode Interactive 3D display mode Học - -CH HỌC-8-gCH Interactive 3D display mode ana CH HC-9 aaa Interactive 3D display mode CH HCC-CH yi CH₃ Submit Previous Answers Request. Which of the following pairs are positive isomers. Compounds in the third pair are metamers.
Compounds in the first pair are position isomers. Propyne and cyclopropene are ring chain isomers as one isomer possesses an open chain structure while other has cyclic structure. Ii Aniline and ethylamine.
Option B is the correct answer. DEthyl alcohol and ethylene glycol. 1-Propanol and methoxyethane have the same molecular formula but different functional.
Underset Butane-2-ol CH_ 3underset OHunderset CHCH_ 2CH_ 3 underset 2-Methylpropan-2-ol CH_ 2underset CH_ 3underset overset OHoverset CCH_ 3 Which of the following pairs of compounds. In this manner isomers contain a similar number of iotas for every component except the nuclear game plan varies. Get Answer to any question just click a photo and upload the photo and get the answer completely free.
Give one chemical test to distinguish between the following pairs of compounds. Select the correct response 1-butene 2-butene 2-methylpropene cyclobutene 2-butene cyclobutene 2-methylpropene 1-butene. What is the relationship between the members of the following pairs of structures gt Are they indentical structural or geometrical isomers or reson asked May 27 2019 in Chemistry by xD4y4.
In number 4 both compounds contains three carbon atoms one oxygen and 6 hydrogen atoms that makes them isomers. The answer is number 4. A and B and C HC C - CH 3 and CH 3 - C CH D and E and Answer.
A CH3 H H H CH3 CH3 H CC CH and C H and H H CH3 CH3 B CH3 H СН2СН3 C HC C- CH3 and CH3 - C CH D CH3 H н CH3 CC CH3 CH3 CH3 H and CH3 H H CH3 and E H H H сHз 3. They are functional isomers. C H 3 C H 2 C H 2 C O C H 3 and C H 3 C H 2 C O C H 2 C H 3 are the Position Isomers.
2-methylbutane and 2 2-dimethylbutane. Submit Answer Retry Entire Group 6 more group. B 24 The reaction of hydrogen H 2.
Propanal and Propanone are functional isomers of each other with the functional group CO. The following pair of compounds which can be classified as positional isomers is. Which of the following pairs of compounds represents skeletal isomers.
Alcohols exhibiting chain isomerism have the same position of the funtional group but possess different skeletons. Which of the following pairs of compounds are structural isomers. Which of the following pairs of compounds are not position isomers.
E 23 What is the name of the compound shown below. The first compound is an ester CH_3CH_2COOCH_3 while the second one is alpha- methoxyketone CH_3COCH_2OCH_3. Alcohol R O H and Ethers R O R are functional isomers as they have the same molecular formula but the different functional group.
A 2 - pentene B trans - 2 - pentene C trans - 3 - pentene D cis - 2 - pentene E cis - 3 - pentene Answer. We have to know that isomers are two atoms with a similar subatomic recipe however vary fundamentally. 2 Which of the following pairs of compounds are constitutional isomers.
Chemistry questions and answers. Florianmanteyw and 2 more users found this answer helpful. Ethanoic acid and ethane-1 2-diol.
Which of the following pairs of compounds are not position isomers. Propyne and propadiene allene are functional isomers. Propene and cyclopropene are not isomers as they differ in their molecular formula.
Tell whether the following pairs of compounds are identical constitutional isomers stereoisomers or unrelated. A and CHCHCH B cl Br and x C and ست and E None of these pairs are constitutional isomers 6 What is the major organic product of the following reaction of. The Pair Of Compounds that Are Isomers are CH3COCH3 and CH3CH2CHO.
Find three pairs of isomers from the compounds given below. The following pair of compounds which can be classified as positional isomers is. This browser does not support the video element.
Note that cis trans isomers are an example of stereoisomers.

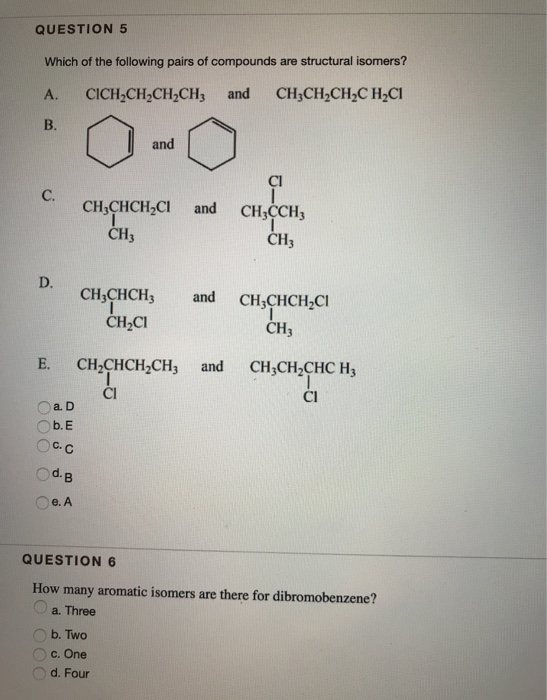
Solved Question 5 Which Of The Following Pairs Of Compounds Chegg Com
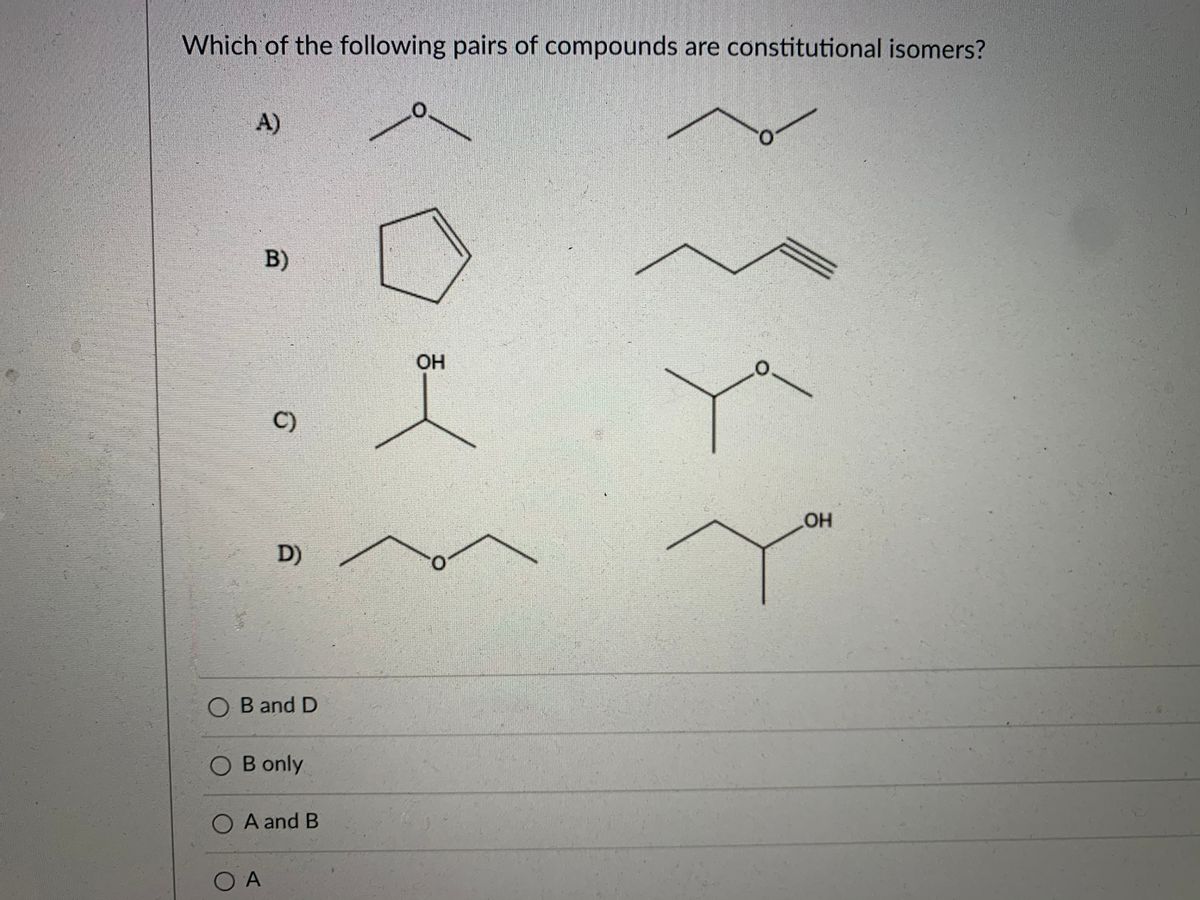
Answered Which Of The Following Pairs Of Bartleby

Organic Chemistry Educational Infographics Isomers Organic Chemistry Chemistry Educational Infographic
No comments for "Which of the Following Pairs of Compounds Are Isomers"
Post a Comment